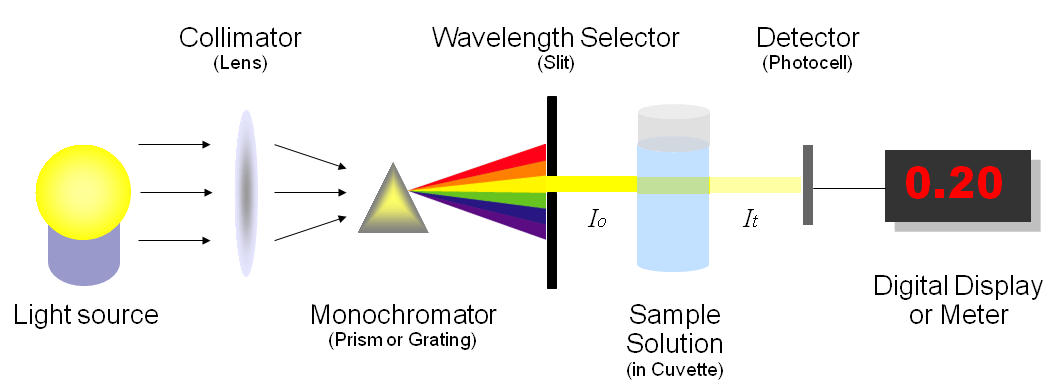
Spectrometry and spectroscopy are related but they are not the same thing. Spectrometry is the application of spectroscopy; spectrometry creates quantifiable results for later assessment.
Once you understand the basics of spectrometers, you will need to understand exactly how it measures and provides results before you can analyze the data the equipment offers. While a good bit of the data is relatively straightforward, the units of measurement for a spectrometer may take a bit more knowledge to fully understand it. The units of measurement aren’t terribly difficult to grasp, but the results are not presented in a way that you have likely encountered elsewhere.
What a Spectrometer Measures
To understand the units of measurement, you have to understand what a spectrometer is measuring.
Spectrometry is a method of spectroscopy, which means that spectrometry is quantifying the amount of energy absorbed by matter and the light that it creates in the process. Essentially, each substance will either transmit or absorb light, and the frequency by which the substance does this identifies what the substance is.
Spectrometers measure the frequency emitted by the substance being analyzed. Since it clearly is not something that can be measured as easily or as simply as units of distance or weight, it does have its own units to determine that frequency.
Many types of spectrometers use radiation (such as x-rays) to cause a reaction from the sample that will identify its composition. These types of reactions cannot be determined simply by watching the reaction because they are often not visible to the human eye. This means that the measurements are done through the tools.
Measuring Units for Absorption
Given that the measurement of light includes methods that clearly go beyond what the human eye can see, the units of the spectrometer reflect the complexity of what is being measured.
Wave Length
The wavelength of light absorption or transmittance is measured based on the wavelength in nanometers. While the unit seems straightforward, a machine is required because the human eye cannot accurately detect the frequency of the absorption or transmittance of such a minuscule length.
Light Intensity
The spectrometer also provides results on the intensity of the light. This requires the use of several complex formulas to calculate the spectral transmittance of the sample or object being examined.
Although it covers more than just the information on spectrometers, the Chemistry Libre Texts provides a very detailed breakdown and details on how spectrophotometry measures light, including refraction.
© Chemistry Libre Text
If you are interested in learning about the more scientific aspects and uses for the results, this site can help you better understand the results and how to interpret them across a wider range of fields. It may not be necessary to understand all of the math behind the measurements, but for those who are interested it can be exciting. The additional information also gives you a bit of deeper knowledge for how the different methods are better solutions for a variety of needs.
Keep in mind that spectrometers measure a much wider range than just visible light, which is only a fraction of the wavelengths of light. The full wavelength of light goes from the gamma ray (10-5 nanometers) to radio waves (1013 nanometers). Radio waves can be thousands of meters long, while the gamma ray wavelength is not visible to the human eye because it is so miniscule.
The Optical Emissions Spectrometer (OES)

Furthermore, any light that has been generated by the discharge can be said to be a collection of the spectral lines as generated by the elements within the sample. This light can be split by a diffraction grating. This is done in order to extract the emission spectrum for the target elements. The concentration of the element in the sample determines the intensity of each emission spectrum.involve the application of electrical energy in the form of a spark which is generated between an electrode and a metal sample. As a result, the vaporized atoms are brought to a high energy state within what is called the discharge plasma. Then, within the discharge plasma, these excited atoms and ions create a particular emission spectrum which is specific to each element. As a result, a single element can easily end up generating numerous characteristic emission spectral lines.
Detectors (these are photomultiplier tubes) measure the presence or absence of the spectrum as extracted for each element. They also measure the intensity of the spectrum. This is done in order to perform a quantitative and qualitative analysis of the elements.
OES can also be referred to as Atomic Emission Spectrometry, although that name also connotes flame emission spectroscopy. OES, in contrast, is a kind of ‘spark and arc’ used to analyze the metallic elements contained within solid samples of an alloy. The general purpose is to measure and thereby perform production quality control.
Typical users of OES Services include foundries and metal casting facilities, as they use metals and alloys all the time, and the purity of metals and alloys is of very high importance.
Conclusion
While the machines and tools used to measure absorption tend to be accurate, they still require a certain level of maintenance and calibration to ensure they remain reliable. From initial testing to repairs and maintenance, you can contact us about the PMI and OES services we offer.
We can help you to ensure your tools and equipment continue to function as intended.